By Patrick Holford

Eating a Mediterranean style diet with lots of vegetables and fruit keeps your brain 18 years younger, shows a new study published on the 8th March 2023 in Neurology. According to the researchers “People who scored highest for adhering to the Mediterranean diet had average plaque and tangle amounts in their brains similar to being 18 years younger than people who scored lowest.” They also found people who scored highest for adhering to the MIND diet had average plaque and tangle amounts similar to being 12 years younger than those who scored lowest.”
Adding just one food category from either diet — such as eating recommended amounts of vegetables or fruits — reduced amyloid build-up in the brain to a level similar to being about four years younger, the study said. The greatest result was found with those eating greens. Those in the top third of ‘greens consumption’ had substantially less Alzheimer’s related pathology that those in the lowest third – not eating their greens.
“Doing a simple dietary modification, such as adding more greens, berries, whole grains, olive oil and fish, can actually delay your onset of Alzheimer’s disease or reduce your risk of dementia when you’re growing old,” said study author Puja Agarwal, an assistant professor of internal medicine at the Rush University Medical Center in Chicago.
“Those with a healthy diet have seven times less risk of developing dementia compared to those eating an average diet, according to a study last month in the British Medical Journal” says Patrick Holford, who directs the Alzheimer’s prevention project at Food for the Brain Foundation, “This is completely consistent with Food for the Brain’s free on-line Dementia Risk Index questionnaire which assesses a person’s risk and asks specific questions about diet, including eating greens. Their test includes a Cognitive Function Test which measures actual cognition so you can gauge your brain age. It then advises you what to do to keep your brain young and healthy.”
Read ‘The Alzheimer’s Prevention Diet’ and discover six brain-friendly recipes including these protective foods.
By Patrick Holford
Taking vitamin D supplements may help ward off dementia, according to a new, large-scale study involving over twelve thousand dementia-free 70+ year olds in the US. More than a third (37%) took supplements of vitamin D. After adjusting for baseline age, sex, education, race, cognitive diagnosis, depression, and APOE4 status, exposure to prescribed vitamin D supplements was associated with 40% lower incidence of dementia during a ten-year period.
The study, carried out by researchers from the University of Calgary’s Hotchkiss Brain Institute in Canada and the University of Exeter in the UK, has been published in the journal Alzheimer’s & Dementia. The team found that taking vitamin D was associated with living dementia-free for longer, and they also found 40 per cent fewer dementia diagnoses in the group who took supplements.
Professor Zahinoor Ismail, of the University of Calgary and University of Exeter, who led the research, said: “We know that vitamin D has some effects in the brain that could have implications for reducing dementia, however so far, research has yielded conflicting results. Our findings give key insights into groups who might be specifically targeted for vitamin D supplementation. Overall, we found evidence to suggest that earlier supplementation might be particularly beneficial, before the onset of cognitive decline.”
One of the problems in relation to vitamin D and cognition is the use of variable amounts of vitamin D, and the lack of reporting regarding blood levels at baseline and conclusion of trials. This study has the same issue. We do not know the dose taken nor the starting and end blood levels of those supplementing or not.
A review by Dr William Grant, Director of Sunlight, Nutrition and Health Research Center, who is the expert on our Scientific advisory Board shows that the higher the blood level of vitamin D (25-hydroxyvitamin D [25(OH)D] is the form measured in the blood serum) the lower is the risk of developing Alzheimer’s as well as dementia in general. The mechanisms identified as potentially explaining its benefit include reduced brain aging, cellular senescence, mitochondrial dysfunction and oxidative stress as well as higher high-density lipoprotein (HDL). Observational study findings indicate that blood levels above 30 ng/mL (75 nmol/L) reduce dementia risk by about 40% and Alzheimer’s risk by about 30% compared to those with blood levels below 12 ng/mL (30 nmol/L) which is broadly consistent with the scale of benefit reported in this trial. You can read Dr William Grant’s full review entitled “The role of vitamin D in reducing risk of Alzheimer’s disease” here.
“In this study, while Vitamin D was effective in all groups, the team found that the beneficial effects were significantly greater in females, compared to males. Similarly, effects were greater in people with normal cognition, compared to those who reported signs of mild cognitive impairment – changes to cognition which have been linked to a higher risk of dementia.
Dr William Grant says “It is unlikely that vitamin D supplementation is of much use for treating advanced stages of these diseases, although it would be useful in reducing risk of other vitamin D-sensitive adverse health outcomes.” Overall, he says “The evidence regarding vitamin D satisfies the criteria for causality in a biological system for reducing risk of cognitive function, Alzheimer’s and vascular dementia. Thus, vitamin D supplementation can be recommended as an additional way to reduce risk of these diseases. It should also be useful for reducing the rate of progression of these diseases.”
The mechanism(s) for explaining the beneficial effects of vitamin D are not yet clear, however one interesting finding was that the effects of vitamin D were also significantly greater in people who did not carry the APOEe4 gene, known to present a higher risk for Alzheimer’s dementia, compared to non-carriers. The authors suggest that people who carry the APOE4 gene absorb vitamin D better from their intestine, which might reduce the vitamin D supplementation effect due to higher baseline vitamin D levels. However, no blood levels were drawn to test this hypothesis.
Co-author Dr Byron Creese, at the University of Exeter, said: “Preventing dementia or even delaying its onset is vitally important given the growing numbers of people affected. The link with vitamin D in this study suggests that taking vitamin D supplements may be beneficial in preventing or delaying dementia, but we now need clinical trials to confirm whether this is really the case. The ongoing VitaMIND study at the University of Exeter is exploring this issue further by randomly assigning participants to either take vitamin D or placebo and examining changes in memory and thinking tests over time.”

At Food for the Brain those taking the Cognitive Function Test also answer questions about their use, and level of vitamin D, if known. We hope, soon, to start offering vitamin D testing to further research the effects of vitamin D in relation to cognitive decline to be able to track progress both cognitive changes against vitamin D blood levels and intake, to help determine an optimal daily intake or blood level. We are also working with the charitable Grassroots Health Nutrient Research Institute who have tested thousands of people’s blood vitamin D level and are now encouraging them to take Food for the Brain’s Cognitive Function Test. You can join their Vitamin D action group here.
Listen to Patrick Holford’s podcast explaining the role of vitamin D in Alzheimer’s disease here.
Originally published on Orthomolecular Medicine News Service [15th February 2023]
In the days of Hippocrates, diseases were blamed on the gods. He didn’t buy that and explored the causes of disease saying ‘let food be thy medicine’. Nowadays a lot of diseases are being blamed on genes – because knowledge about genes and their effects has advanced tremendously over the last several decades. Genes are the code, or instructions, to assemble proteins, for example to make an enzyme, a hormone or a biochemical such as cholesterol or phospholipids.
Take Alzheimer’s, which accounts for two thirds of dementia, as an example. There are only three genes that can cause Alzheimer’s (APP, PSEN1, PSEN2), and these account for considerably less than one in a hundred cases of Alzheimer’s. [1]
There are, however, 76 other genes [2] which appear to confer a very small additional risk. Taken together, estimates suggest that 75-85% of the risk can be explained by combining these into a polygenic risk score. [3] The single greatest predictor is the presence of the ApoE4 variant of the ApoE gene, carried by about one in five people. It is considered to contribute 4 to 6% of the absolute risk for Alzheimer’s disease. [4,5]
This is often exaggerated as a risk factor because, if a person has the ApoE4 gene, and changes nothing, they have about a 20% greater chance of developing Alzheimer’s later in life than someone who doesn’t. This is called ‘relative risk’. It doesn’t mean, however, that someone with the ApoE4 gene has a 20% chance of developing Alzheimer’s. This is because, as an example, a person without the ApoE4 gene at a certain age might have a 5% chance of developing Alzheimer’s, while someone with the ApoE4 gene might have a 6% chance, so their risk has gone up by, in this example, 20%. In absolute terms, the risk would be only 1% higher.
Predicting risk and actually reducing risk with modifications of diet and lifestyle are two different things. The predictive risk for Alzheimer’s of having a low intake of seafood and/or omega-3 fats is 22%, and so is having a low intake of B vitamins resulting in a high blood homocysteine level. Smoking confers a similar risk. [6] Other big risk factors are an inactive lifestyle and low level of education. Add in predictive genes and apparent risk adds to well over 100% partly because there is overlap.
But the only way to find out how much you can actually reduce a person’s risk by is to either conduct ‘observational’ studies looking at, e.g. smokers vs non-smokers, or people with a good versus a bad diet, and see how many develop dementia. Even better is to change something, such as looking at what happens when a person stops smoking, or supplements omega-3 fish oils or homocysteine lowering B vitamins.
All these so-called Alzheimer’s genes, with the exception of the causative ones, can only exert effects via non-genetic mechanisms and these mechanisms are often susceptible to modification with a person’s nutrition having the most direct influence. In other words, gene variants that are present are not either active or inactive. Even if you have a gene variant such as ApoE4 it is more like a dimmer switch and can be ‘over-expressed’ or ‘down-regulated’, turned up or dimmed down. That is why approximately half of women with the BRCA gene develop breast cancer and half don’t. The environment the gene is exposed to makes all the difference.
The expression and harmful effects of the ApoE4 gene appear to be downregulated by eating a low-glycemic load (GL) diet or a more ketogenic diet with specific Mediterranean-style food choices including fatty fish, cruciferous vegetables, olive oil, and low alcohol consumption. Six supplemental nutrients have reasonably good evidence of down-regulating ApoE4. These are omega-3 DHA, B vitamins (B2, B6, B12 and folate) vitamins D3 and K2, quercitin and resveratrol. [7] This approach to modifying the effects of the genes we inherit with personalised nutrition is a fundamental tenet of orthomolecular medicine, sometimes called personalised, precision or optimum nutrition.
But what happens to risk if a person is doing these things already? A good example of this is a recent study in China, involving 29,072 people of which 20% had the ApoE4 gene. [8] Each participant had their diet and lifestyle assessed over the 10 year period of the study to see who would or wouldn’t develop cognitive decline or dementia.
The study showed that whether or not a person had the ApoE4 ‘Alzheimer’s gene’ made no difference to the positive reduction in risk achievable by simple diet and lifestyle changes. “These results provide an optimistic outlook, as they suggest that although genetic risk is not modifiable, a combination of more healthy lifestyle factors is associated with a slower rate of memory decline, regardless of the genetic risk,” wrote the study authors.
Eating a healthy diet was the most important prevention step, followed by an active lifestyle, with one’s intellectual life, then physical activity, then social interactions being the next most important steps. Eating a healthy diet was about twice as important as exercise in predicting cognitive decline. Those with a healthy diet were about seven times less likely to have age-related cognitive decline or dementia than those with an ‘average’ diet and about nine times less likely to develop dementia than those with an unfavorable diet.
The assessment of a healthy diet was based on intake of fish, eggs, fruits, vegetables, legumes, nuts and tea, among other foods known to predict lower risk.
Other Alzheimer’s related genes affect a process called methylation. Healthy methylation depends on adequate B vitamin intake, primarily B6, B12 and folate. Inheriting a variant of a key methylation gene, MTHFR 677TT increases risk for Alzheimer’s. [9-11] About one in three people have this gene variant. It impacts risk by raising homocysteine, a toxic amino acid that damages the brain and blood vessels. Having a raised homocysteine level increases risk for cerebrovascular dysfunction 17-fold. [12]
Since methylation is needed to make phospholipids, biochemicals essential for the brain also found in eggs and fish, having a poor diet in this respect creates more methylation demand and, consequently, greater need for B vitamins.
In a placebo controlled study of older people with mild cognitive impairment, about a third of participants had the MTHFR variant that increases Alzheimer’s risk. But supplementing with B vitamins effectively lowered homocysteine in both those with and without this ‘Alzheimer’s’ gene. The B vitamin supplement almost arrested further memory decline and slowed the rate of brain shrinkage by 52%, [13,14] reducing shrinkage of the Alzheimer’s areas of the brain by 9-fold. [15] Whether a person did or didn’t have this ‘Alzheimer’s’ gene made no difference to the beneficial effect of the B vitamins.
Those with adequate omega-3 blood levels had even less brain shrinkage – 73% less than the placebo group. [16-17] Two other studies have found major protection either by giving B vitamins to those with adequate omega-3 intake, [18] or by supplementing omega-3 to those with lower homocysteine levels [19] further confirming that you need both B vitamins and omega-3 fats to keep neurons healthy – an example of synergy – regardless of one’s genes. Whether a person did or didn’t have the MTHFR variant made no significant difference.
Too often genes are blamed as drivers of disease even though (with the exception of rare causative genes) the primary drivers are what you put in your mouth or how you live your life – both factors under our control. For example, DNA genetic testing can cause panic when an individual is informed they have a dozen or more gene variants. Over-emphazing the importance of genes discourages people from preventing their own disease by improving diet and lifestyle.
You can find out what’s driving your risk and which diet and lifestyle changes will make the biggest difference by doing the Cognitive Function Test at foodforthebrain.org and joining COGNITION, the brain upgrade program. Not only do you help yourself, you also help the hundreds of thousands of people who would benefit from the research we support at Food for the Brain to reduce risk of dementia.
(Patrick Holford , BSc, DipION, FBANT, NTCRP is widely published and a member of the Orthomolecular Medicine Hall of Fame. He is the director of the non-profit, UK-based “Alzheimer’s is Preventable” campaign [ foodforthebrain.org].)
1. Bekris LM, Yu CE, Bird TD, Tsuang DW. (2010) Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol. 23:213-227. https://pubmed.ncbi.nlm.nih.gov/21045163
2. Bellenguez C, Küçük F, Jansen IE, et al. (2022) New insights into the genetic etiology of Alzheimer-s disease and related dementias. Nat Genet. 54:412-436. https://pubmed.ncbi.nlm.nih.gov/35379992
3. Escott-Price V, Myers AJ, Huentelman M, Hardy J. (2017) Polygenic risk score analysis of pathologically confirmed Alzheimer disease. Ann Neurol. 82:311-314. https://pubmed.ncbi.nlm.nih.gov/28727176
4. Heininger K (2000), A unifying hypothesis of Alzheimer’s disease. III. Risk factors. Hum Psychopharmacol Clin Exp. 15:1-70. https://pubmed.ncbi.nlm.nih.gov/12404343
5. Ridge PG, Mukherjee S, Crane PK, Kauwe JSK, (2013) Alzheimer’s Disease: Analyzing the Missing Heritability. PLoS One. 8(11): e79771. https://pubmed.ncbi.nlm.nih.gov/24244562
6. Beydoun MA, Beydoun HA, Gamaldo AA, et al. (2014) Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 14:643. https://pubmed.ncbi.nlm.nih.gov/24962204
7. Norwitz NG, Saif N, Ariza I.E, Isaacson RS (2021) Precision Nutrition for Alzheimer’s Prevention in ApoE4 Carriers. Nutrients 13:1362. https://pubmed.ncbi.nlm.nih.gov/33921683
8. Jia J, Zhao T, Liu Z et al. (2023) Association between healthy lifestyle and memory decline in older adults: 10 year, population based, prospective cohort study. BMJ 380:e072691. https://pubmed.ncbi.nlm.nih.gov/36696990
9. Morris AA, Kožich V, Santra S, et al. (2017) Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis. 40:49-74. https://pubmed.ncbi.nlm.nih.gov/27778219
10. Bouguerra K, Tazir M, Melouli H, Khelil M. (2022) The methylenetetrahydrofolate reductase C677T and A1298C genetic polymorphisms and plasma homocysteine in Alzheimer’s disease in an Algerian population. Int J Neurosci. 29:1-6. https://pubmed.ncbi.nlm.nih.gov/36580407
11. Zuin M, Cervellati C, Trentini A, et al. (2021) Methylenetetrahydrofolate reductase C667T polymorphism and susceptibility to late-onset Alzheimer’s disease in the Italian population. Minerva Med. 112:365-371. https://pubmed.ncbi.nlm.nih.gov/32700867
12. Teng Z, Feng J, Liu R, et al. (2022) Cerebral small vessel disease mediates the association between homocysteine and cognitive function. Front. Aging Neurosci. 14:868777. https://pubmed.ncbi.nlm.nih.gov/35912072
13. Smith AD, Smith SM, de Jager CA, et al. (2010) Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 5(9):e12244. https://pubmed.ncbi.nlm.nih.gov/20838622
14. Smith AD, Refsum H. (2016) Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr. 36: 211-239. https://pubmed.ncbi.nlm.nih.gov/27431367
15. Douaud G, Refsum H, de Jager CA, et al. (2013) Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci USA 110:9523-9528. https://pubmed.ncbi.nlm.nih.gov/23690582
16. Jernerén F, Elshorbagy AK, Oulhaj A, et al. (2015) Brain atrophy in cognitively impaired elderly: the importance of long-chain omega-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. 102:215-221. https://pubmed.ncbi.nlm.nih.gov/25877495
17. Oulhaj A, Jernerén F, Refsum H, et al. (2016) Omega-3 fatty acid status enhances the prevention of cognitive decline by B vitamins in Mild Cognitive Impairment. J Alzheimer’s Dis. 50:547-557. https://pubmed.ncbi.nlm.nih.gov/26757190
18. van Soest, A.P.M., van de Rest, O., Witkamp, R.F. et al. (2022) DHA status influences effects of B-vitamin supplementation on cognitive ageing: a post-hoc analysis of the B-proof trial. Eur J Nutr. 61:3731-3739. https://pubmed.ncbi.nlm.nih.gov/35704085
19. Jernerén F, Cederholm T, Refsum H, et al. (2019) Homocysteine Status Modifies the Treatment Effect of Omega-3 Fatty Acids on Cognition in a Randomized Clinical Trial in Mild to Moderate Alzheimer’s Disease: The OmegAD Study. J Alzheimers Dis. 69:189-197. https://pubmed.ncbi.nlm.nih.gov/30958356
By Patrick Holford
One theory was that it was to do with the accumulation of amyloid protein, producing amyloid plaque that interferes with brain cell communication. But, despite over 30 clinical trials, lowering amyloid protein has had close to zero clinical effect. But, even if this was part of the problem, one would have to ask why?
There are plenty of left-field theories. One, for example, is that it’s an auto-immune disease whereby the brain starts to destroy itself. There are plenty of diet and lifestyle diseases that tip over into auto-immune diseases. For example, type-2 diabetes can convert to type-1 diabetes and osteoarthritis can convert to rheumatoid arthritis. But even so, one would have to ask why? What would be driving this?
There are over 20 known risk factors that predict future risk for cognitive decline, dementia and/or Alzheimer’s. These include:
So far researchers have looked at individual known risk factors for Alzheimer’s, then tried to change them with some success. The nutritionists have tried to change diet, or give supplements. The pharmacologists have tried to give drugs to lower, for example, high blood pressure or insulin levels. The psychologists have tried to increase cognitive stimulation and address depression and isolation, and insomnia. The sports physiologists have tried to increase exercise. But what do all these factors have in common? Is there a way of looking that ties all these risk factors together into an understanding as to what is actually driving dementia?
The old, but still dominant mindset in science is ‘reductionism’. The idea is to look at one thing, or one risk factor, then change it in a randomised placebo-controlled trial. The idea is that if everyone does this then you could pool all the interventions together to produce a cure. This reminds me of a comment made in the G8 summit on dementia in 2010, in London, when we succeeded in getting a discussion on dementia prevention added to the agenda. The pharma representative said words to the effect of ‘we will solve dementia with multiple drugs, just like we solved AIDS’. The reality is studies giving drugs[1] to lower blood sugar for diabetics, lower blood pressure with anti-hypertensive drugs, lower cholesterol with statins, even lower amyloid protein, have failed. The official cost of all this research is $42.5 billion to date.[2] This is five times more than the cost of the James Webb telescope. This approach clearly isn’t working. The only ‘drugs’ that have worked are homocysteine lowering B vitamins and omega-3 fish oils – and the recent discovery is that they work together, in cooperation.
There’s a new emerging way of doing science which is called ‘systems-based’ science. The physicist, Fritjof Capra, has explained this way of doing science in his book ‘The Web of Life’. He says “Systems thinking emerged from a series of interdisciplinary dialogues among biologists, psychologists, and ecologists, in the 1920s and ’30s. In all these fields, scientists realized that a living system—organism, ecosystem, or social system—is an integrated whole whose properties cannot be reduced to those of smaller parts. The “systemic” properties are properties of the whole, which none of its parts have. So, systems thinking involves a shift of perspective from the parts to the whole. The early systems thinkers coined the phrase, “The whole is more than the sum of its parts.”[3]
Us humans are a complex adaptive system. What’s also been learnt about complex adaptive systems is that they have a certain amount of ‘resilience’ which you can think of as the credit in your health deposit account. When that runs out, disease occurs. Many leaders in the field of nutritional and naturopathic medicine consider that many of the same underlying processes are going wrong in our bodies, which then cause the emergence of a ‘disease’ depending on the organ it strikes – so heart disease, diabetes, arthritis and dementia have similar contributing factors.
At Food for the Brain, we can organise all these risks above into eight domains shown below. It is certainly true that these eight domains of ‘risk’ cover much of what we know about the risks for heart disease, diabetes and arthritis, for example. This partly, but not fully. answers the question about what is actually driving dementia.
Another understanding within systems-based thinking is well illustrated by asking the question ‘what is the difference between an inanimate object, like a bicycle, and an animate organism, such as us?’ A bicycle has ‘parts’ and the parts related to each other, as in functioning together. So do we. But also, there is ‘life’ running through us. You can imagine your brain’s neural network lighting up, with ‘energy’ or signals shooting this way and that. If you have healthy parts, all functioning, but no signals, you’re kind of ‘switched off’.
We call the parts – structure; the relationship of the parts – function; and the life running through the neural network – utilisation. These are shown visually below in a way to illustrate that they are integral, with each dependent on the other.
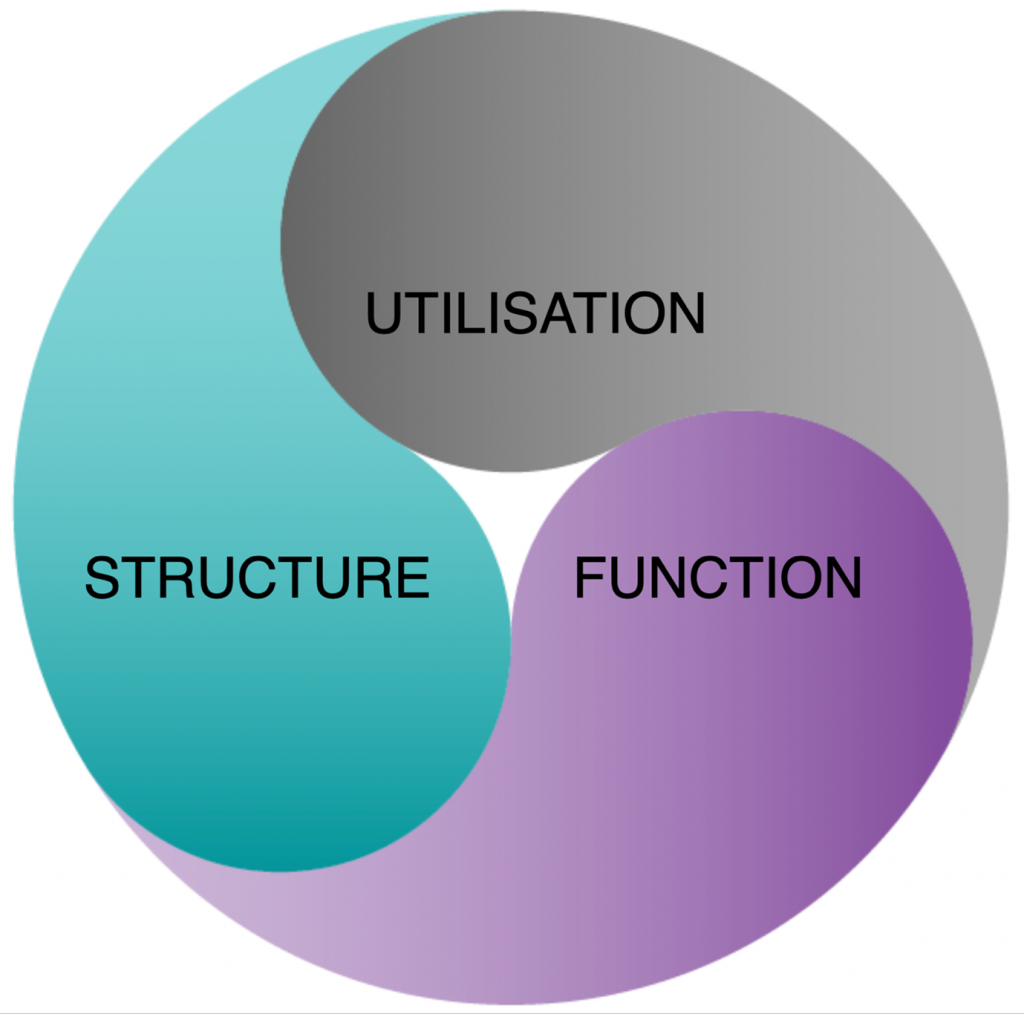
Now, let’s reorganise all those risk factors accordingly into whether they are primarily required for the structure or function of the neural network, or send messages across it.
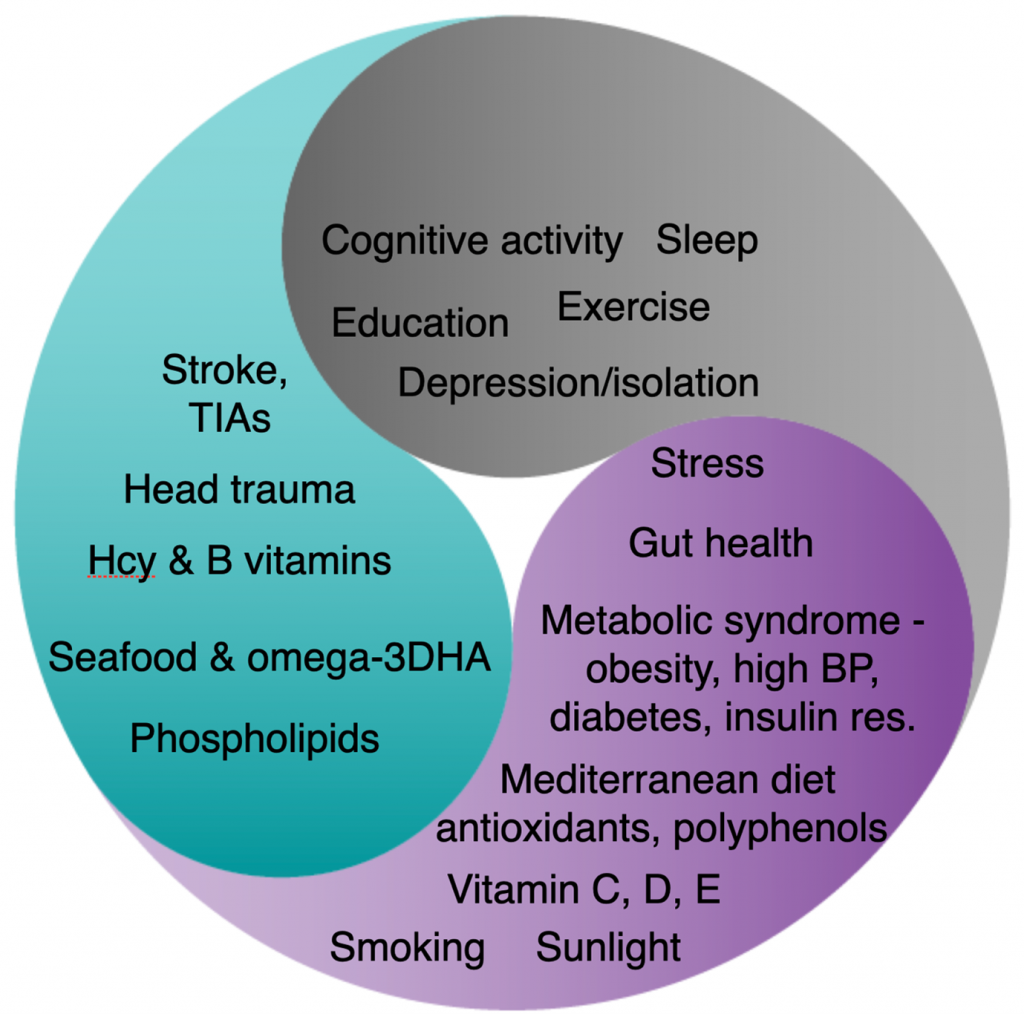
If you’ve watched the short animated film ‘how to keep building brain cells at any age‘ you’ll know that the membrane of every brain cell is made by binding omega-3 DHA, rich in seafood, to phospholipids and especially Phosphatidylcholine, rich in eggs and fish, to produce what called ‘phosphorylated DHA’. You need this to have a functioning brain. It actually makes up more than 90 per cent of the structure of your brain.
You’ll also know that the ‘binding’ of these two parts depends on B vitamins, which drive a process called methylation. So, without enough B vitamins your brain and nervous system fall apart. The best measure of methylation, and whether you are getting enough B vitamins, is your blood homocysteine level. If your homocysteine level is high, you’ve got a problem. Above 11mcmol/l and you’ve got a shrinking brain. More than half of all people over 70 have a homocysteine level above this. It should, therefore, be no surprise to find that, if you have a raised homocysteine level, and are given B vitamins, if you also have a low omega-3 DHA intake or blood level, the B vitamins won’t work. Conversely, if you supplement omega-3 fish oils, but have a raised homocysteine level or lack of B vitamins, the omega-3 won’t work. The full story of the dynamic duo of Omega-3 and B vitamins is explained here.
It is likely that choline deficiency, which is especially common in those who rarely eat fish or eggs, may also create a similar structural problem in the brain. In animals supplementing choline prevents Alzheimer’s related brain changes.[4]
There are many aspects of ‘function’ of the brain. Using a simple car analogy, one thinks of the need for fuel and the need for oil to lubricate the parts. The two main fuels of brain cells are either glucose, derived from carbs, or ketone derived from fat.
If you’ve watched the animated film ‘how to fuel your brain for better memory’, you’ll know about the need for slow-releasing carbs and ketones from a type of fat called a medium chain triglyceride (MCT) and specifically C8 oil.
Too much sugar, and especially fructose and high fructose corn syrup (now used by the food industry to sweeten foods), and too much refined carbohydrates interferes with fuel supply to brain cells by making you ‘insulin resistant’. Insulin receptors, embedded in neuronal membranes, transport glucose into brain cells. If these receptors, which are like doors, are largely shut down, the brain starves of clean fuel. Read professor Robert Lustig’s article “Is sugar killing your brain?’ for the full story.
The alternative brain fuel – ketones, which neurons actually prefer, can be made in the liver from a type of fat called C-8 (caprylic acid triglyceride) which makes up 7 per cent of coconut oil. In a study giving people with memory problems two tablespoons of C8 oil, their brains produced 230 per cent more energy from ketones and their memory improved. The article ‘Is fat the best brain fuel?’ gives you the full story.
The lubricating ‘oil’ in a car analogy would be both dealing with the ‘exhaust fumes’ of the brain’s energy production, namely oxidants. These are mopped up by antioxidants and polyphenols rich in plant foods. That where food such as blueberries and cacao, or vitamin C and E, come in. It’s also why smoking is such a big risk factor.
Another part of ‘function’ is circulation – anything that improves circulation helps the function. Many of the things we’ve mentioned – lowering homocysteine with B vitamins, omega-3, antioxidants, polyphenols – also help circulation.
Another part of ‘function’ is inflammation. Behind all those ‘metabolic’ diseases -diabetes, heart disease, arthritis to name a few – lies inflammation which doesn’t just affect the specific organ, be it heart of joint, but also the brain.
One of our experts, Tommy Wood, assistant research professor at the University of Washington in Seattle, focussing on neuroscience, has developed an excellent model for understanding the ‘use it or lose it’ principle. He’s big into exercise.
‘Exercise is important because it makes the brain do things that keep it healthy, such as growth and repair and maintaining temperature and weight,’ he says. ‘When they aren’t stimulated, the health of brain tissues deteriorates with a knock-on effect on memory and thinking.’
And it’s not just physical exercise that does this, we also benefit from the mental exercise involved in likes of solving puzzles or learning a new language. ‘For many people the worst thing they can do for their brain is to retire’, says Wood. ‘They lose much of the stimulation that kept it healthy.’
Sleep as a brain protector also fits in here. It’s vital for recovering from both physical and intellectual exercise and to store and organise what you have learnt in the day.
‘But sleep and exercise aren’t enough on their own,’ Wood continues. ‘All that repair and maintenance needs a good supply of nutrients.’
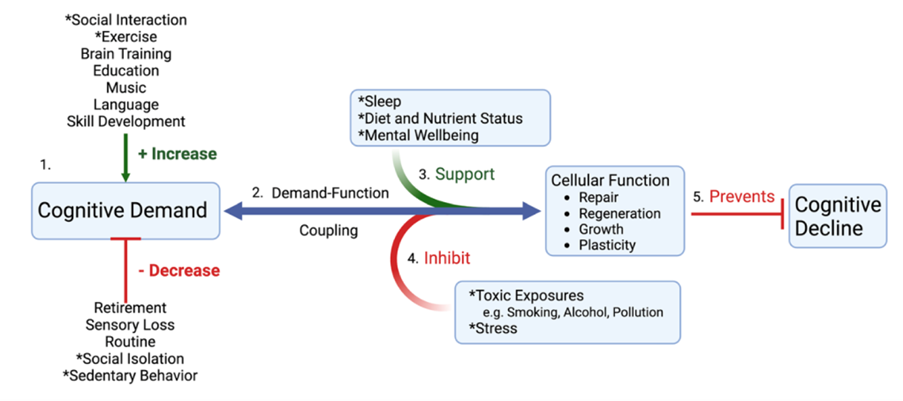
Stress also fits in here because stress, as well as environmental and dietary ‘pollution’ be it from drinking and smoking, dirty air, moulds, even allergens like gluten with can induce ‘brain fog’ often experienced by those with coeliac disease, promote inflammation and inhibit repair and regeneration.
This systems-based approach to what’s potentially driving cognitive decline makes it obvious that there will never be a single drug or single factor that stops a person developing dementia. Instead, if a person has enough ‘interference’ with the structure, function or utilisation of their brain then there will, inevitably be cognitive decline with age.
At Food for the Brain, when you complete your Cognitive Function Test, you know objectively how you are doing and how much room for improvement there is. Then you are invited to complete the Dementia Risk Index questionnaire, which not only gives you a score out of 100% (you are aiming for a score closer to 0%) but also shows you in which domain you have the most room for improvement.
You are then invited to join COGNITION, which is an interactive brain upgrade programme that targets your weakest areas and shows you the simplest changes that will make the biggest difference to reduce your risk.
The goal is to turn all your reds, oranges and yellows into green, then reassess your cognitive function. By joining you are becoming part of a group of hundreds of thousands of citizen health scientists helping to discover what really works to dementia-proof your diet and lifestyle.
Take the FREE Cognitive Function Test today.
[1] Peters R, Breitner J, James S, Jicha GA, Meyer PF, Richards M, Smith AD, Yassine HN, Abner E, Hainsworth AH, Kehoe PG, Beckett N, Weber C, Anderson C, Anstey KJ, Dodge HH. Dementia risk reduction: why haven’t the pharmacological risk reduction trials worked? An in-depth exploration of seven established risk factors. Alzheimers Dement (N Y). 2021 Dec 8;7(1):e12202. doi: 10.1002/trc2.12202. PMID: 34934803; PMCID: PMC8655351.
[2] Cummings JL, Goldman DP, Simmons-Stern NR, Ponton E. The costs of developing treatments for Alzheimer’s disease: A retrospective exploration. Alzheimers Dement. 2022 Mar;18(3):469-477. doi: 10.1002/alz.12450. Epub 2021 Sep 28. PMID: 34581499; PMCID: PMC8940715.
[3] Fritjof Capra (2009) The New Facts of Life: Connecting the Dots on Food, Health, and the Environment, Public Library Quarterly, 28:3, 242-248, DOI: 10.1080/01616840903110107
[4] Velazquez R, Ferreira E, Knowles S, Fux C, Rodin A, Winslow W, Oddo S. Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell. 2019 Dec;18(6):e13037. doi: 10.1111/acel.13037. Epub 2019 Sep 27. PMID: 31560162; PMCID: PMC6826123.
Responding to the ‘feverish media coverage heralding a new era of disease modifying treatments’, described by the BBC as a ‘momentous breakthrough’ a scathing editorial in the British Medical Journal says: “Hyperbolic rhetoric gives patients and their families false hope, which clinicians must address, and pre-empts regulatory decision making.”
“Such treatment has been long hoped for,” they say. “However, the null effects on cognition of other anti-amyloid agents, the tiny effect on cognition reported for [the new drug] lecanemab and concerns about safety mean that perspective is needed.”
“The prevailing narrative is that this trial “succeeded” where others have “failed.” In reality, lecanemab, like other anti-amyloid agents, successfully cleared amyloid from the brain. This clearance had no discernible effect on cognition in some trials, a very small and non- significant effect in other trials, and a very small significant effect in the latest trial. The overall trial evidence tells us that successful amyloid clearance in adults with early Alzheimer’s disease has either no effect or a tiny effect on cognitive decline.”
“Previous attempts to quantify the minimum clinically important difference in the trial’s primary outcome measure—the Clinical Dementia Rating (CDR) sum of boxes score (range 0-18 —suggested that minimum changes of 0.98 in mild cognitive impairment and 1.63 in mild Alzheimer’s disease are meaningful. After 18 months of treatment with lecanemab, differences of 0.35 and 0.62 for those with mild cognitive impairment and mild Alzheimer’s disease, respectively, fell well short, representing only around a third of what a minimum clinically important difference might look like.”
Both B vitamins and omega-3 have achieved a clinically significant reduction in the CDR by these criteria, as well as improving other measures of cognition, and in reducing the rate of brain shrinkage. The rate of brain shrinkage reduction of this kind of drug is 2% compared to up to 73% less shrinkage with B vitamins in those with sufficient omega-3. Yet both UK, US and EU government and medical agencies have repeatedly declined funding a definitive trial of both B vitamins and omega-3.
The BMJ editorial expresses serious concerns about safety of this class of drug, which is really an antibody injection. “As with other anti-amyloid agents, lecanemab comes with substantial safety concerns. During the trial, 12.6% of participants treated with lecanemab developed brain oedema (swelling), 22% of whom were symptomatic. A further 17.3% experienced brain haemorrhage; and 6.9% experienced adverse events severe enough to discontinue the trial.” That means that 30% of drug trial participants had a serious adverse effect.
While the number of deaths in the main trial were comparable between the drug and placebo group “more information is needed about two deaths reported during the trial’s open label extension. Both participants had brain haemorrhage, possibly associated with taking lecanemab alongside anticoagulants or thrombolysis.”
They say that “Lecanemab if licensed is likely to cost tens of thousands of pounds a year for each patient. In addition, health systems would need to provide PET scans or lumbar puncture to determine eligibility, fortnightly infusions of the drug indefinitely, and repeated MRI [scans] to monitor for adverse events, all of which is far beyond the capacity of most countries, even those with well-resourced healthcare systems.” B vitamins and omega-3 have no side-effects, other than knock-on health improvements, and cost pennies, not thousands of pounds.
Pressure for approval and clinical use, the BMJ says, is likely to be fierce. “Viewed objectively, however, lecanemab is not the hoped for “game changer.” Rather, it is further evidence that anti-amyloid therapies do not produce clinically meaningful benefits for people with Alzheimer’s disease. Weighed against the scale and severity of adverse events and substantial practical barriers to widespread use, lecanemab is unlikely to represent a favourable risk-benefit balance for patients or value for money for health systems.”
The fully referenced BMJ editorial can be viewed here.
If you are concerned about age-related cognitive decline, dementia or Alzheimer’s please take our free, validated Cognitive Function Test here and sign up to join our COGNITION programme, to help dementia-proof your diet and lifestyle. Also, please support our work in helping teach people who to prevent dementia by becoming a FRIEND of Food for the Brain here.
A hugely significant study in the British Medical Journal into age-related cognitive decline and dementia has stated that changing your diet and lifestyle from bad to good cuts your future risk of developing dementia by a massive nine times [1]
The study shows, significantly, that whether or not you inherit the ApoE4 ‘Alzheimer’s gene’ that one in five people carry, it makes no difference to the positive reduction in risk achievable by simple diet and lifestyle changes [2].
Eating a healthy diet was also the most important prevention step, followed by an active lifestyle, with one’s intellectual life, then physical activity, then social interactions being the next most important steps. Eating a healthy diet was about twice as important as exercise in predicting cognitive decline.
This study, published on 25th January 2023, followed over 30,000 people over a decade and found that those with a healthy diet were about seven times less likely to have age-related cognitive decline or dementia than those with an ‘average’ diet and about nine times less likely to develop dementia than those with an unfavourable diet.
The assessment of a healthy diet was based on intake of fish, eggs, fruits, vegetables, legumes, nuts and tea, among other foods known to predict lower risk.
“These results provide an optimistic outlook, as they suggest that although genetic risk is not modifiable, a combination of more healthy lifestyle factors are associated with a slower rate of memory decline, regardless of the genetic risk,” wrote the study authors.
This study has been warmly welcomed by charity Food for the Brain, as it backs up their own research and the work they have been actively carrying out for 10 years, to help people reduce their risk of age-related cognitive decline.
Food for the Brain offers a free online assessment of a participant’s diet and lifestyle, called the Dementia Risk Index, which works out a person’s overall risk. The assessment also includes a cognitive function test to assess your memory. This charitable ‘citizen science’ action group have also just launched COGNITION, an interactive, personalised ‘brain upgrade’ programme that then shows you, week-by-week, how to make positive changes to bring your risk closer to zero.
Indeed, the on-line test assesses all the same risk factors the British Medical Journal study has shown impact a person’s future risk – diet, active physical, intellectual, social lifestyle, smoking and drinking habits.
According to Professor David Smith from the University of Oxford, one of the charity’s scientific advisors, “Genes can only exert effects via non-genetic mechanisms and these mechanisms are often susceptible to modification by, for example, improving one’s diet. This study shows that diet and lifestyle are much more important than inheriting a gene variant such as ApoE4. Less than 1% of Alzheimer’s is directly caused by genes. With no clinically effective drugs, and minimal role of genes, this study confirms that the focus must be on making diet and lifestyle changes that reduce risk of developing dementia, as foodforthebrain.org are doing. It also shows that switching from an average to a healthy lifestyle, with positive diet changes being key, can dramatically reduce a person’s future risk of developing cognitive decline and dementia.”
Another member of the science team, Dr Celeste de Jager Loots, Research Associate at Imperial College’s AGE Unit where she researches risk factors and prevention of Alzheimer’s and dementia, explains that “While having inherited certain genes can be used to predict risk of dementia, that risk is changed by making positive diet and lifestyle changes. The emphasis needs to be on changing diet and lifestyle, especially since one cannot change one’s genes. The earlier one starts with a healthy lifestyle the better the chance of preventing effects of genetic risk.”
Risk for dementia can be detected from the age of 35 and subtle changes, picked up by Food for the Brain’s cognitive function test, can be seen up to 40 years before a diagnosis. The charity wants anyone over 35 to take the test and start making positive diet and lifestyle changes. “The average person can cut their future risk by three quarters just by making simple diet and lifestyle changes.” says Patrick Holford, who is directing the Alzheimer’s prevention project. “This prevention approach, if we reach enough people, could cut cases of dementia in the UK by a third. That’s why we are urging everyone over 35 to tell everyone they know to take the free and scientifically – validated and free Cognitive Function Test. Alzheimer’s dementia, which accounts for the vast majority of dementia, is irreversible. But it is preventable, as this study shows.”
Two thousand people every month are joining this campaign, assessing and reducing their risk. Over 380,000 people have now taken the test.
Jia, J. et al. (2023) “Association between healthy lifestyle and memory decline in older adults: 10 year, population based, prospective Cohort Study,” BMJ [Preprint]. Available at: https://doi.org/10.1136/bmj-2022-072691. [1]
The evidence that only 1% of Alzheimer’s is caused by genes is here: Bekris, L.M. et al. (2010) “Review article: Genetics of Alzheimer Disease,” Journal of Geriatric Psychiatry and Neurology, 23(4), pp. 213–227. Available at: https://doi.org/10.1177/0891988710383571. [2]
The free Cognitive Function test and Dementia Risk Index assessment is accessed here: https://smartkidsandteens.foodforthebrain.org.
For more information on the citizen science campaign see ‘Alzheimer’s is Preventable – A Manifesto for Change’.
Patrick Holford, founder of Food for the Brain and voluntary director of the Alzheimer’s Prevention Project and Jessica Ferrari-Wells, Chair of the Board of trustees are available for interview, as are members of the the Scientific Advisory Board are available for interview and comment.
Neurons, that is brain and nerve cells, are primarily made out of what’s called ‘phosphorylated DHA’. That means the omega-3 fat DHA that is bound to a kind of fat called a phospholipid, as shown in the figure below.
Seafood contains phosphorylated DHA but DHA supplements, whether derived from fish oil or algae, is not phosphorylated. Hence, it needs to be attached to phospholipids to work. This attachment is done by a B vitamin dependent process called methylation.
There are several different kinds of phospholipids with strange names all starting with ‘phosphatidyl’ such as phosphatidyl choline, phosphatidyl serine, phosphatidyl inositol and phosphatidyl ethanolamine. To a large extent these can be made from phosphatidyl choline. As a group of nutrients they are classified as ‘semi-essential’ because we can make some, but not enough for optimal health and especially optimal brain health.
As a consequence there are moves afoot to classify choline (which can be easily attached to the ‘phosphatidyl’ part) as an essential nutrient with a recommended intake. This has come about due to the growing evidence that insufficient choline in pregnancy leads to cognitive impairment and developmental delay. This is particularly important for vegans because, like the omega-3 fatty acid DHA, there’s not much choline in plant-based foods, but there is some in foods such as quinoa, soya, beans, nuts and broccoli.
Currently an adequate intake of choline is defined as between 400mg and 520mg a day, the latter for pregnant and breast-feeding women. This is based on how much choline you need for healthy fat metabolism, liver function and reducing homocysteine levels. You also need choline to process cholesterol in the liver and brain. As you’ll see in the figure above, cholesterol is a vital brain component. But these levels don’t take into account what’s being learnt about choline’s role in brain development.. A good estimate of optimum daily choline intake would be at least 500mg and maybe double this in pregnancy.
Most important is choline’s role in building, and maintaining, a healthy brain. A pregnant woman’s intake defines the cognitive abilities of their child. Twenty years ago we knew that pregnant rats fed choline half way through their pregnancy have more connections between brain cells, plus improved learning ability and better memory recall. Now we know it’s true for babies with several recent trials showing similar results indicating that more choline in pregnancy enhances cognitive development.
An example of this is a study which gave women in their third trimester of pregnancy either 480mg of choline or almost double this – 930mg. They then tested the babies’ information processing speed at 4,7,10 and 13 months. Not only were the babies of the mothers given the higher dose faster but also the longer the mother had been given even the lower dose the faster were the child’s reactions. The authors concluded that “even modest increases in maternal choline intake during pregnancy may produce cognitive benefits for offspring ”. Seven years later, there will still memory advantages in the children whose mother had extra choline during pregnancy.
Babies are born with blood choline levels three times higher than their mother, illustrating how vital this nutrient is for building neuronal connections, which newborn babies do at a rate of up to a million new connections a second! An optimal intake for brain function is likely to be a lot higher than the 400 to 500mg recommended for adults, and higher still in pregnancy.
Since brain cells are made of a membrane containing choline (and other phospholipids) attached to the omega-3 fat DHA, without choline the omega-3 doesn’t work. The attaching of the two depends on methylation, a process that is dependent on B vitamins, especially B12, folate and B6. Choline helps methylation and healthy methylation, indicated by a low blood level of homocysteine, helps synthesize choline. You need all three – DHA, choline and B vitamins especially B12. So, if you are lacking in DHA, or in vitamin B12, then you’ll be doubly dependent on getting enough choline.
While the richest dietary sources are fish, eggs and organ meats there is significant amounts of choline in plant-based foods, notably soya as in tofu and soya milk, quinoa, nuts and seeds including flax seeds, almonds and peanuts, and cruciferous vegetables including broccoli, cauliflower and Brussels sprouts.
While, on the face of it, it does appear than vegans, especially those planning pregnancy, need to become choline focused in relation to choosing the right daily foods, and possibly supplementing, there is not yet conclusive evidence showing that vegan mothers are at risk, although it is likely that they are. One of the learnings that has come out of studies on omega-3 DHA is than vegan mothers may convert more vegan omega-3 ALA into DHA as an evolutionary imperative – not that a top up with supplementation isn’t still the recommendation. Could it be that vegan mothers make more choline if needed since it is so important for brain development? There are very few studies of vegans to know the answer to this question.
One recent study looked at choline levels in breast-milk of vegans, versus vegetarians and non-vegetarians. There was no significant difference with the author of the study concluding “This suggests that maternal plant-based diet by itself is not a risk factor for low breast-milk choline.”
The vegan community is certainly divided on this issue. Of course, the safe or cautious position, while the science unravels, is to supplement choline during pregnancy.
What intake of choline can you achieve from a vegan diet alone? Here’s a list of the best plant-based food for choline, compared to egg and fish as a yardstick, listed in order of how much you could get in a reasonable serving*:
FOOD CHOLINE PER SERVING PER 100g
An egg (all in the yolk) 50g 113mg 226mg
Fish eg salmon (100g/3oz) 90mg 90mg
Soya milk (cup – 250g) 57mg 23mg
Shiitake mushrooms (1 cup/145g) 54mg 37mg
Soya flour 12.5g (a cake slice) 24mg 192mg
Peas (1 cup -160g) 47mg 30mg
Quinoa, raw (1/3 cup 60g) 42mg 70mg
Beans, raw (1/3 cup – 60g) 40mg 67mg
black, white, pinto, kidney
Broccoli, cauliflower
or sprouts (1 cup/91g) 36mg 40mg
Tofu (half a cup-125g) 35mg 28mg
Hummus (1/2 cup) 34mg 28mg
Chickpeas (1/4 can) 33mg 33mg
Baked beans (1/4 can) 31mg 31mg
Flaxseeds (small handful) 22mg 78mg
Pistachio (small handful) 20mg 71mg
Pine nuts (small handful) 18mg 65mg
Cashews (small handful) 17mg 61mg
Wholegrain bread (2 slices – 50g) 17mg 34mg
Avocado (1/2) 14mg 28mg
Almonds 50g (small handful) 12mg 42mg
Peanuts (small handful) 12mg 42mg
Wheatgerm (tablespoon 7g) 12mg 178mg
Almonds or peanut butter (tbsp) 10mg 61mg
Source: USDA choline content database and https://nutritiondata.self.com
*Many foods have not been analysed for choline, and measurements do vary, so this is a guide rather than a definitive list.
What does this mean for your daily diet? Here’s a typical vegan daily menu aimed to maximise choline intake and how much it would give you (I’m not including all foods and recipes, just those ingredient that deliver significant amount of choline):
BREAKFAST
A cup of soya milk 57mg
Small handful of nuts or seeds 20mg
(Flax, chia, almonds etc)
LUNCH
A cup of cooked quinoa (1/3 cup raw) 43mg
A serving (100g) of either broccoli, 36mg
cauliflower or Brussels sprouts
Avocado (1/2) 14mg
SNACKS
A tablespoon of almond or peanut butter 10mg
Hummus (1/2 cup) 34mg
Two slices of wholegrain bread 17mg
DINNER
A serving of tofu (125g) or beans 35-40mg
Half a cup of shiitake mushrooms 27mg
A serving (100g) of either broccoli, 36mg
cauliflower or Brussels sprouts
TOTAL 332mg
In reality you are unlikely to achieve this every day, and it would be quite limiting on your food choices, so a realistic target would be to achieve 300mg of choline from food. If you are aiming to achieve 500mg, which is the low end of optimal – more than this may be optimal in pregnancy – that leaves a shortfall of around 200mg of choline, suggesting the need for supplementation.
The most direct source of choline is from soya-derived lecithin granules and capsules. A flat tablespoon of lecithin granules (7.5g), which has a neutral and pleasant taste and can be sprinkled on cereals, in shakes and soups or eaten as is, provides 1,500 mg of phosphatidylcholine and around 200mg (13 per cent) of choline. Some ‘high phosphatidyl choline’ lecithin, sometimes called ‘high PC lecithin’ is 18 per cent choline, thus you need less – approximately a flat dessertspoon.
One tablespoon of lecithin granules equals three 1,200mg lecithin capsules (if ‘high PC’ two capsules would suffice). We suggest that this is a sensible addition to a completely vegan diet. (If you aspire to be plant-based most, but not all of the time the addition of two eggs, or an egg and a fish serving, would achieve 500mg a day of choline.)
You can also find ‘brain food’ supplements providing a combination of different kinds of phospholipids, not just choline, but its hard to get enough choline from these if your only other food sources are plant-based foods.
In summary, we need both omega-6 and omega-3 fats, as well as phospholipids.
If you are not completely vegan the best food source for phospholipids and choline are eggs. Eat six eggs a week. The choline is in the yolk. The advice regarding omega-3 – eat three servings of fish a week, is good for choline too but it is present in all fish, not just oily fish high in omega-3 fats.
Have you taken the Cognitive Function Test to find out your Dementia Risk Index score? It’s completely FREE and you can choose to pay for the COGNITION programme afterwards if you need personalised recommendations to help you put diet and lifestyle tips into action.
The BBC’s recent story headed ‘Dementia: Brain check-up tool aims to cut risk at any age’ is a step in the right direction since early prevention is the key to reducing risk. However, the online brain health check, hosted by the Alzheimer’s Research Trust, is very basic, with only a dozen questions, and ignores the key evidence-based and common nutritional risk factors for Alzheimer’s disease. It covers ‘stay sharp’ which is about mental stimulation, ‘stay connected’ which is about social interaction and ‘love your heart’.
It ignores the two strongest nutritional risk factors, namely homocysteine lowering B vitamins and omega-3 intake from seafood. The US National Institute for Health’s research [1] attributes 22% of Alzheimer’s risk to each of these.
‘Love your heart’ gives advice to keep your cholesterol and blood pressure in check and manage diabetes. This refers an individual to their GP who is likely to prescribe statins to lower cholesterol and hypertensive drugs to lower blood pressure. Neither reduce Alzheimer’s risk. A recent major review [2] of the evidence concludes ‘prospective, randomized, placebo‐controlled clinical trials that have failed to provide evidence for the benefit of statin therapy’ and there is ‘insufficient [evidence] to tell us whether reducing BP for dementia risk reduction is effective.’
The same review recommends omega-3 supplementation is start early and maintained and B vitamin supplementation to lower homocysteine, which is cited as the most evidence-based prevention approach considered. This report says “In view of the high population attributable risk, it is important that raised homocysteine can readily be lowered by the oral administration of three B vitamins (folate, B6, and B12). The doses of these vitamins that are required to lower homocysteine are considerably larger than can readily be obtained from the diet.” This is based on evidence of the VITACOG trial [3] which was part funded by the Alzheimer’s Research Trust.
A GP could lower a person’s risk much more effectively by measuring homocysteine in the blood and recommending B vitamin supplements, as well as upping omega-3 intake by eating fish and/or supplementing. This combination has reduced the rate of brain shrinkage by up to 73% in those with pre-dementia [4] but only few GPs do.
The Think Brain Health check does not assess diet, or make specific recommendations, and there’s no mention of protective supplements, but refers people to the NHS’s ‘eight tips for healthy eating’. These recommend ensuring starchy carbohydrates make up over a third of what you eat, reducing saturated fat and salt. There is no evidence that these reduce risk for Alzheimer’s. Too many carbs may actually be a promoter of cognitive decline. They also recommend eating less sugar, lots of fruit and veg and having at least 2 portions of fish a week, including at least 1 portion of oily fish. There is evidence that these recommendations may reduce dementia risk.
In sharp contrast foodforthebrain.org’s validated Cognitive Function Test and Dementia Risk Index questionnaire (139 questions) both objectively measure a person’s cognitive function and calculates their risk, then gives specific and actionable instructions as to how a person can reduce their risk, inviting them back every six months to track their progress.
The Cognitive Function Test is free and for those who wish to improve, we have launched COGNITION, a new way to Upgrade your Brain at a low cost of just £5 per month. You can access both tests once logged into your account.
1. Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014 Jun 24;14:643. doi: 10.1186/1471-2458-14-643. PMID: 24962204; PMCID: PMC4099157.
2. Peters R, Breitner J, James S, Jicha GA, Meyer PF, Richards M, Smith AD, Yassine HN, Abner E, Hainsworth AH, Kehoe PG, Beckett N, Weber C, Anderson C, Anstey KJ, Dodge HH. Dementia risk reduction: why haven’t the pharmacological risk reduction trials worked? An in-depth exploration of seven established risk factors. Alzheimers Dement (N Y). 2021 Dec 8;7(1):e12202. doi: 10.1002/trc2.12202. PMID: 34934803; PMCID: PMC8655351.
3. Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM, Jacoby R, Refsum H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010 Sep 8;5(9):e12244. doi: 10.1371/journal.pone.0012244. PMID: 20838622; PMCID: PMC2935890.
4. Oulhaj A, Jernerén F, Refsum H, Smith AD, de Jager CA. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J Alzheimers Dis. 2016;50(2):547-57. doi: 10.3233/JAD-150777. PMID: 26757190; PMCID: PMC4927899.
A recent study of 1,178 women found that those carrying the APOE4 gene taking Hormone Replacement Therapy (HRT) had a better delayed memory score compared to APOE4 carriers that were not taking HRT, and to non-APOE4 carriers.[1] They also had slightly larger brain volumes in certain areas. This study suggested that HRT may help to prevent Dementia. This study was an observational trial, not a clinical trial, meaning the statement remains a hypotheses and requires further randomised controlled trials to investigate further. We analysed the paper and provided our comments below.

Clinical trials to date have not shown benefit of HRT with improving cognitive function. A systematic review of the clinical trial evidence for the effect of HRT on cognitive outcomes did not find benefit.[2] The Women’s Health Initiative Memory Study (WHIMS) conducted a double-blind, placebo-controlled clinical trial examining 8300 women 65 years of age or older over a 2- year period to observe the effects of HRTs and dementia progression. The trial failed to find a beneficial effect for HRT in reducing dementia risk, instead finding an increase in all types of dementia.[3]
Roughly 1 in 5 people carry the ApoE4 gene, which accounts for 4 to 6% of risk for dementia and can be modified, downregulating the gene, with positive diet, nutritional supplement and lifestyle changes.[1]
In our Dementia Risk Index, as part of the Cognitive Function test, and COGNITION programme to reduce dementia, we excluded HRT because the evidence was not conclusive or consistent.
Have you tried our free Cognitive Function Test yet? Find out your Alzheimer’s disease risk using our evidence backed Dementia Risk Index. If your risk is high, our clever new programme COGNITION can help you make the right nutrition and lifestyle changes to help improve your score.
[1] Saleh RNM, Hornberger M, Ritchie CW, Minihane AM. Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimers Res Ther. 2023 Jan 9;15(1):10. doi: 10.1186/s13195-022-01121-5. PMID: 36624497; PMCID: PMC9830747.
[2] Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1(1):CD004143.
[3] Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in post- menopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651-2662.